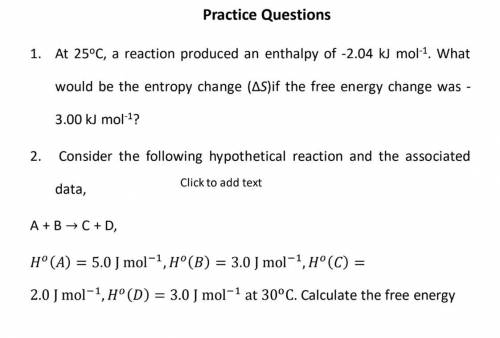
25oC, a reaction produced an enthalpy of -2.04 kJ mol-1
. What
would be the entropy change (Δ...

Chemistry, 01.08.2021 17:00 Homepage10
25oC, a reaction produced an enthalpy of -2.04 kJ mol-1
. What
would be the entropy change (ΔS)if the free energy change was -
3.00 kJ mol-1?
2. Consider the

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3


Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1
You know the right answer?
Questions

Mathematics, 03.05.2020 14:15

History, 03.05.2020 14:15

English, 03.05.2020 14:15

Mathematics, 03.05.2020 14:15

History, 03.05.2020 14:15

Mathematics, 03.05.2020 14:15

Mathematics, 03.05.2020 14:15


Mathematics, 03.05.2020 14:15


Mathematics, 03.05.2020 14:15





Mathematics, 03.05.2020 14:15

Mathematics, 03.05.2020 14:15

Mathematics, 03.05.2020 14:15

Mathematics, 03.05.2020 14:15



