
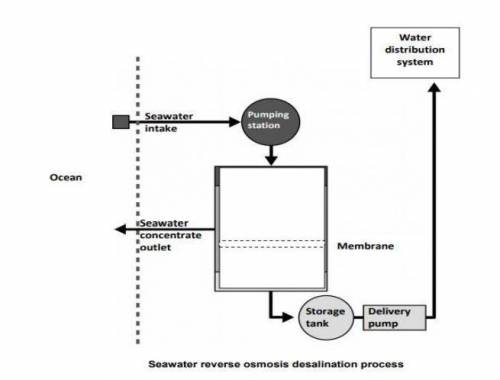
One way of producing drinking water from seawater is by reverse osmosis.
Reverse osmosis is a type of filtration. Seawater is pushed through a semi-permeable membrane. Pressure is applied to the seawater. Semi-permeable
means salt is trapped on one side of the membrane, but water can pass through.
The trapped salts form a ‘seawater concentrate’ on one side of the membrane.
Where do the trapped salts go?
[a] ocean [b]Water distribution system
[c]Storage tank [d]delivery pipeline
QUESTION 3:
Based on the above picture what will determine whether a particle is able to pass
through the membrane?
[a] particle size [b] particle mass
[c] number of particles [d] charge on the particle

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

You know the right answer?
One way of producing drinking water from seawater is by reverse osmosis.
Reverse osmosis is a type...
Questions

Mathematics, 28.09.2019 04:30

Mathematics, 28.09.2019 04:30


History, 28.09.2019 04:30



Mathematics, 28.09.2019 04:30

Social Studies, 28.09.2019 04:30



Biology, 28.09.2019 04:30

History, 28.09.2019 04:30

Biology, 28.09.2019 04:30

History, 28.09.2019 04:30

Arts, 28.09.2019 04:30




Social Studies, 28.09.2019 04:30



