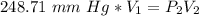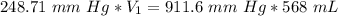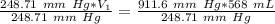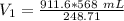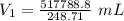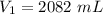Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

You know the right answer?
A sample of oxygen occupied 568 ml. when the pressure increased to 911.6 mm Hg. at constant temperat...
Questions

English, 20.01.2020 20:31

Mathematics, 20.01.2020 20:31






Mathematics, 20.01.2020 20:31




Social Studies, 20.01.2020 20:31

Mathematics, 20.01.2020 20:31



Mathematics, 20.01.2020 20:31


Mathematics, 20.01.2020 20:31

Mathematics, 20.01.2020 20:31

Health, 20.01.2020 20:31