
Chemistry, 02.08.2021 17:10 andreamarie2004amg
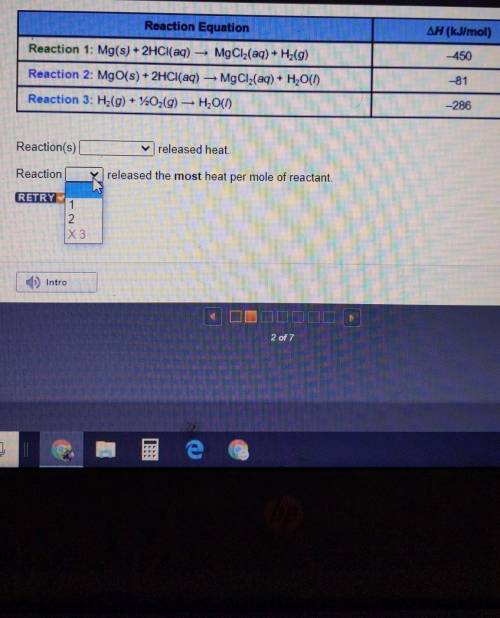
Reaction Equation ∆H (kJ/mol) -450 Reaction 1: Mg(s) + 2HCl(aq) — MgCl2(aq) + Hz(9) Reaction 2: MgO(s) + 2HCl(aq) — MgCl2(aq) + H2O(1) Reaction 3: H2(g) + 220,(9) — H2O(1) -81 -286
Reaction(s)_released heat. W
Reaction_released the most heat per mole of reactant

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Reaction Equation ∆H (kJ/mol) -450 Reaction 1: Mg(s) + 2HCl(aq) — MgCl2(aq) + Hz(9) Reaction 2: MgO(...
Questions





Mathematics, 11.03.2020 23:10

Mathematics, 11.03.2020 23:10

Mathematics, 11.03.2020 23:10



Computers and Technology, 11.03.2020 23:10




Computers and Technology, 11.03.2020 23:10

Mathematics, 11.03.2020 23:10

Mathematics, 11.03.2020 23:10

Mathematics, 11.03.2020 23:10


Mathematics, 11.03.2020 23:10



