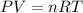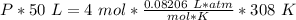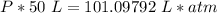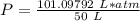Chemistry, 03.08.2021 21:10 rakanmadi87
What is the pressure of 4 moles of helium in a 50 L tank at 308 K?
Use PV = nRT.
A. 24.64 atm
B. 0.13 atm
O C. 0.51 atm
D. 2.02 atm

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
What is the pressure of 4 moles of helium in a 50 L tank at 308 K?
Use PV = nRT.
A. 24.64 atm...
A. 24.64 atm...
Questions


Mathematics, 03.04.2020 01:27


Mathematics, 03.04.2020 01:27


Mathematics, 03.04.2020 01:28

Mathematics, 03.04.2020 01:28





Mathematics, 03.04.2020 01:28

Computers and Technology, 03.04.2020 01:28

Mathematics, 03.04.2020 01:28

Mathematics, 03.04.2020 01:28





Mathematics, 03.04.2020 01:28