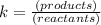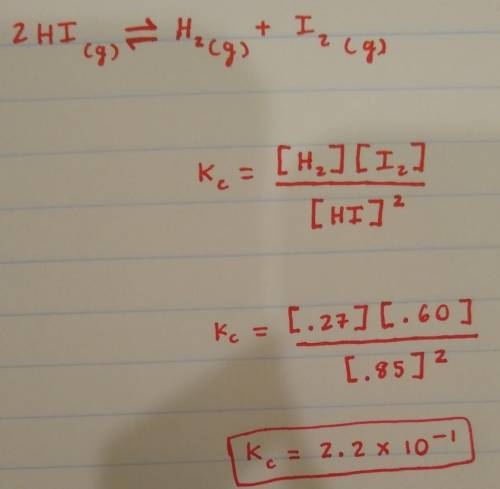Chemistry, 04.08.2021 03:20 salgado100400
At elevated temperatures, hydrogen iodide may decompose to form hydrogen gas and iodine gas, as follows:
2HI(g) ⇌ H2 (g) + I2 (g)
In a particular experiment, the concentrations at equilibrium were measured to be [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, and [H2] = 0.27 mol/L. What is Kc for the above reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
At elevated temperatures, hydrogen iodide may decompose to form hydrogen gas and iodine gas, as foll...
Questions


Mathematics, 15.04.2021 01:50

English, 15.04.2021 01:50


Mathematics, 15.04.2021 01:50


Chemistry, 15.04.2021 01:50

Chemistry, 15.04.2021 01:50




Arts, 15.04.2021 01:50





Mathematics, 15.04.2021 01:50

Spanish, 15.04.2021 01:50