
It is often possible to change a hydrate into an anhydrous compound by heating it to drive off the water (dehydration). A 43.19 gram sample of a hydrate of MgBr2 was heated thoroughly in a porcelain crucible, until its weight remained constant. After heating, 27.21 grams of the anhydrous compound remained. What is the formula of the hydrate?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3


Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
It is often possible to change a hydrate into an anhydrous compound by heating it to drive off the w...
Questions

Mathematics, 01.12.2020 18:40

English, 01.12.2020 18:40

English, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40


History, 01.12.2020 18:40

Biology, 01.12.2020 18:40



Physics, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40

Health, 01.12.2020 18:40

Arts, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40




Mathematics, 01.12.2020 18:40

 .
. denote the number of
denote the number of 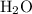 formula units for every
formula units for every  formula unit. The formula of the hydrate would be
formula unit. The formula of the hydrate would be  .
. :
: 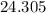 .
. :
: 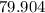 .
. :
:  .
. :
: 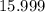 .
. .
. .
.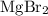 . There was
. There was 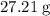 of this compound. Calculate the number of moles of formula units in that much of this compound:
of this compound. Calculate the number of moles of formula units in that much of this compound: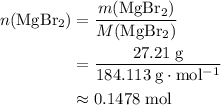 .
.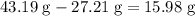 of water
of water 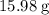 of
of 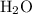 :
: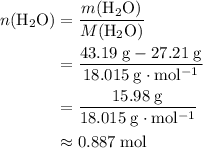 .
. of
of 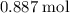 of
of 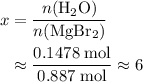 .
.

