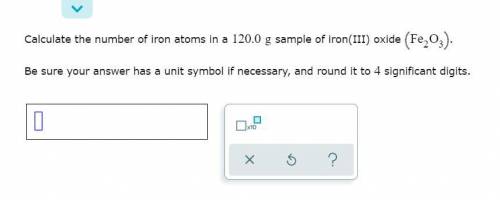
Calculate the number of oxygen atoms in a 140.0 g sample of iron(III) oxide (Fe2O3)?
...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Questions


History, 05.02.2021 07:20

Medicine, 05.02.2021 07:20


History, 05.02.2021 07:20


History, 05.02.2021 07:20

History, 05.02.2021 07:20


Mathematics, 05.02.2021 07:20


History, 05.02.2021 07:20

Chemistry, 05.02.2021 07:20

Mathematics, 05.02.2021 07:20




Mathematics, 05.02.2021 07:20

Mathematics, 05.02.2021 07:20

History, 05.02.2021 07:20