
Chemistry, 07.08.2021 01:00 radaishasmithoxngbj
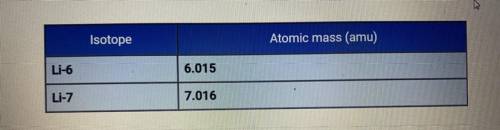
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of lithium, how do the relative abundances of the
isotopes compare?
A. Li-6 is much more abundant than Li-7.
B. They are about the same.
C. Li-7 is much more abundant than Li-6.
D. Li-7 is slightly more abundant than Li-6.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1

Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1

Chemistry, 23.06.2019 15:20
Plzzz ? which stores information in discrete steps? a magnet and coil of wire compact discs plastic records amplified speakers
Answers: 2
You know the right answer?
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of l...
Questions


Mathematics, 28.10.2020 01:50

Mathematics, 28.10.2020 01:50



History, 28.10.2020 01:50



History, 28.10.2020 01:50

English, 28.10.2020 01:50

Health, 28.10.2020 01:50




English, 28.10.2020 01:50





History, 28.10.2020 01:50



