
Chemistry, 07.08.2021 06:20 salinasroel22
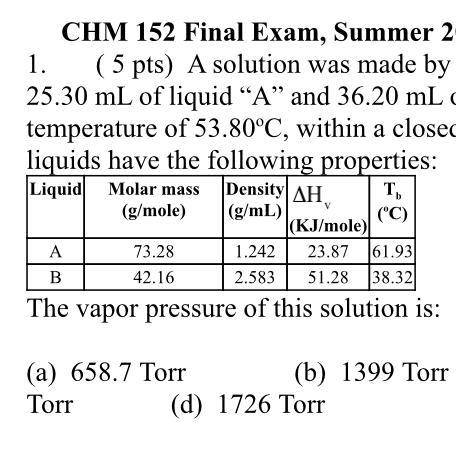
A solution was made by mixing together 25.30 mL of liquid “A” and 36.20 mL of liquid “B” at a temperature of 53.80oC, within a closed container. The liquids have the following properties:
(a) 658.7 Torr
(b) 1399 Torr
(c) 410.7 Torr
(d) 1726 Torr

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
A solution was made by mixing together 25.30 mL of liquid “A” and 36.20 mL of liquid “B” at a temper...
Questions

Mathematics, 22.06.2021 21:50


Computers and Technology, 22.06.2021 21:50


History, 22.06.2021 21:50

Mathematics, 22.06.2021 21:50






Mathematics, 22.06.2021 21:50

Arts, 22.06.2021 21:50


Mathematics, 22.06.2021 21:50







