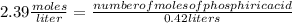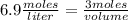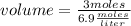Chemistry, 09.08.2021 01:20 ayoismeisjuam
What volume of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid according to
the equation:
H3PO4(aq) + 3NaOH(aq) + Na3PO4(aq) + 3H2O(aq)
A) O 0.05 L
B) O6.93 L
C) O0.44 L
D) 03.01 L
E) 436.43 L


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
What volume of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid according...
Questions



Mathematics, 29.03.2021 20:10



Chemistry, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10



English, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10



Mathematics, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10

History, 29.03.2021 20:10



Geography, 29.03.2021 20:10

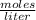 .
.