
Chemistry, 09.08.2021 14:00 ilovecatsomuchlolol
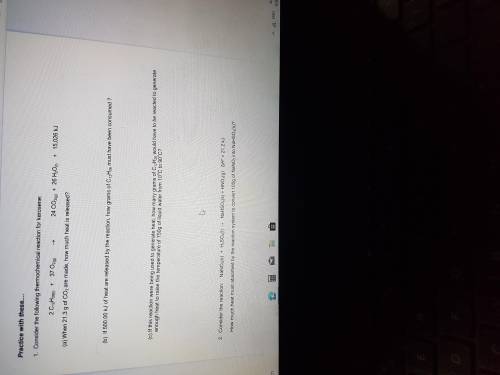
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l)+15,026kj
A. When 21.3g of CO2 are made, how much heat is released ?
B. If 500.00kj of heat are released by the reaction, how many grams of C12H26 must be produced?
C. If this reaction were to being used to generate heat , how many grams of C12H26 would have to be reacted to generate enough heat to raise the temperature of 750g of liquid water from 10 degrees Celsius to 90 degrees celsius ?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l...
Questions



Mathematics, 12.12.2019 19:31




Mathematics, 12.12.2019 19:31


History, 12.12.2019 19:31



Mathematics, 12.12.2019 19:31

Advanced Placement (AP), 12.12.2019 19:31

Physics, 12.12.2019 19:31

Chemistry, 12.12.2019 19:31

Mathematics, 12.12.2019 19:31

Mathematics, 12.12.2019 19:31

Mathematics, 12.12.2019 19:31


History, 12.12.2019 19:31



