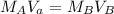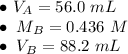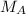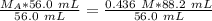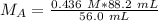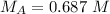Chemistry, 09.08.2021 20:40 ejhoff4347
In a titration experiment, 56.0 mL of an unknown concentration of H3PO4 solution is completely neutralized by 88.2 mL of 0.436 M KOH solution. Calculation the molarity of the acid. Question 11 options: 0.258 M 0.688 M 2.06 M 0.229 M 0.362 M

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
In a titration experiment, 56.0 mL of an unknown concentration of H3PO4 solution is completely neutr...
Questions

Mathematics, 03.09.2021 21:50

Mathematics, 03.09.2021 21:50



Business, 03.09.2021 21:50

Biology, 03.09.2021 21:50

Geography, 03.09.2021 21:50




Mathematics, 03.09.2021 21:50

Social Studies, 03.09.2021 21:50


Mathematics, 03.09.2021 21:50


Mathematics, 03.09.2021 21:50

Mathematics, 03.09.2021 21:50


Mathematics, 03.09.2021 21:50