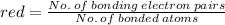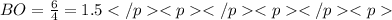Chemistry, 10.08.2021 02:10 vtrvfrfvrvfvnkjrf
The average bond order is the number of bonds between two atoms taking into account resonance.
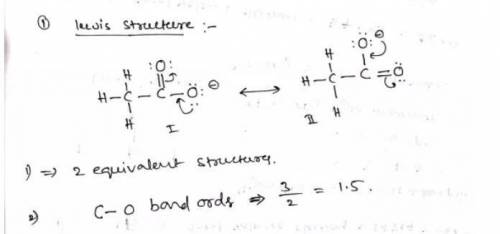
a. Draw a Lewis structure for the nitrite ion and answer the questions below.
1. there are equivalent Lewis structures for NO2-.
2. the average N-O bond order is .
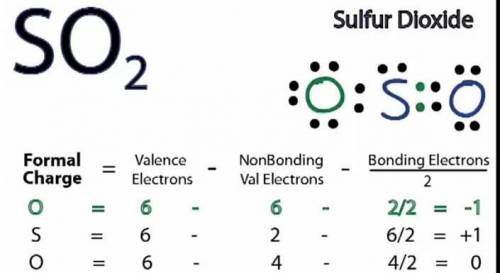
b. Draw a Lewis structure for sulfur dioxide and answer the questions below.
1. there are equivalent Lewis structures for SO2.
2. the average S-O bond order is .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
The average bond order is the number of bonds between two atoms taking into account resonance.
a....
Questions

French, 04.10.2021 07:30





History, 04.10.2021 07:30

History, 04.10.2021 07:30


History, 04.10.2021 07:40

Physics, 04.10.2021 07:40



Mathematics, 04.10.2021 07:40

Mathematics, 04.10.2021 07:40

History, 04.10.2021 07:40

English, 04.10.2021 07:40

Mathematics, 04.10.2021 07:40

Mathematics, 04.10.2021 07:40

English, 04.10.2021 07:40