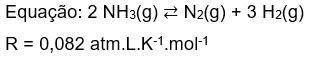Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2
You know the right answer?
4) Considere que em condições de equilíbrio as pressões parciais de NH₃(g), N₂(g) e H₂(g) são respec...
Questions

Mathematics, 15.12.2020 21:10

Physics, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10


Computers and Technology, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10





Geography, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10

History, 15.12.2020 21:10





Mathematics, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10