
Chemistry, 13.08.2021 21:10 tyboutlife
Write the structural formulas for the products of the following three reactions. Name the reaction mechanism.
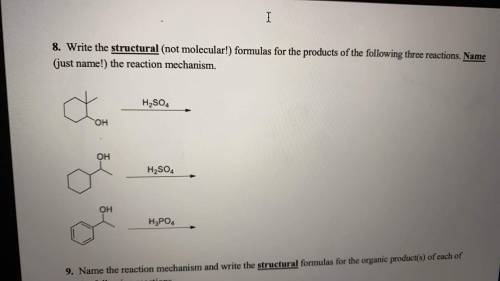

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2
You know the right answer?
Write the structural formulas for the products of the following three reactions. Name the reaction m...
Questions

Biology, 22.10.2019 05:00

Mathematics, 22.10.2019 05:00


Mathematics, 22.10.2019 05:00

Computers and Technology, 22.10.2019 05:00

Mathematics, 22.10.2019 05:00

World Languages, 22.10.2019 05:00

Biology, 22.10.2019 05:00



Mathematics, 22.10.2019 05:00



Social Studies, 22.10.2019 05:00

English, 22.10.2019 05:00

Spanish, 22.10.2019 05:00

English, 22.10.2019 05:00

World Languages, 22.10.2019 05:00

Mathematics, 22.10.2019 05:00

Computers and Technology, 22.10.2019 05:00



