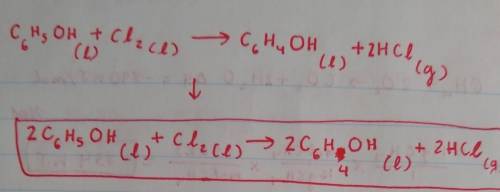Chemistry, 14.08.2021 17:50 kornut7316
A. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products
and medicine. When it reacts with chlorine liquid (chlorine is a diatomic molecule), in the presence of a
catalyst, one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine
atom, and liquid chlorophenol is formed. This replacement process is called chlorination
Write a balanced chemical equation for the chlorination reaction and explain how you balanced it. Note
that hydrogen chloride gas (HCI) is also a product of this chemical reaction and you should ignore the
presence of the catalyst in the equation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
A. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products...
Questions



English, 20.07.2019 15:00

Biology, 20.07.2019 15:00

History, 20.07.2019 15:00




English, 20.07.2019 15:00



History, 20.07.2019 15:00


Social Studies, 20.07.2019 15:00

Mathematics, 20.07.2019 15:00

Biology, 20.07.2019 15:00


History, 20.07.2019 15:00