
Please help!
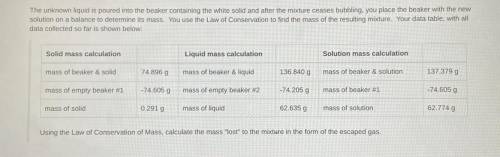
The unknown liquid is poured into the beaker containing the white solid and after the mixture ceases bubbling, you place the beaker with the new
solution on a balance to determine its mass. You use the Law of Conservation to find the mass of the resulting mixture. Your data table, with all
data collected so far is shown below:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1


You know the right answer?
Please help!
The unknown liquid is poured into the beaker containing the white solid and after the...
Questions

History, 11.02.2020 23:56

History, 11.02.2020 23:56

Mathematics, 11.02.2020 23:56







Mathematics, 11.02.2020 23:57

Biology, 11.02.2020 23:57

English, 11.02.2020 23:57

Business, 11.02.2020 23:57

History, 11.02.2020 23:57








