
Chemistry, 23.08.2021 23:30 ThousandSeas9381
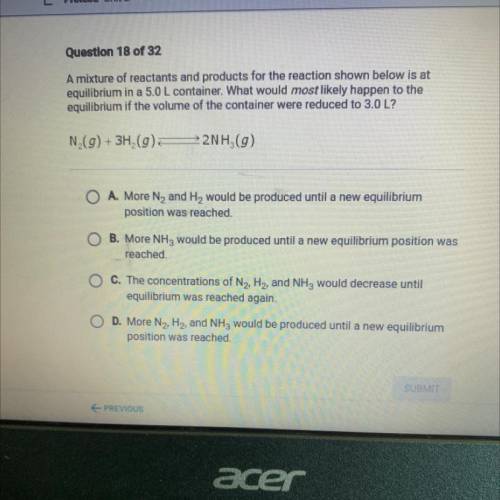
A mixture of reactants and products for the reaction shown below is at
equilibrium in a 5.0 L container. What would most likely happen to the
equilibrium if the volume of the container were reduced to 3.0 L?
N (g) + 3H2(g)
22NH (9)
O A. More N2 and H2 would be produced until a new equilibrium
position was reached.
B. More NH3 would be produced until a new equilibrium position was
reached.
O C. The concentrations of N2, H2, and NH3 would decrease until
equilibrium was reached again.
D. More N2, H2, and NH3 would be produced until a new equilibrium
position was reached.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
A mixture of reactants and products for the reaction shown below is at
equilibrium in a 5.0 L cont...
Questions

History, 18.06.2021 09:30




English, 18.06.2021 09:30

SAT, 18.06.2021 09:30

Mathematics, 18.06.2021 09:30

English, 18.06.2021 09:30


Social Studies, 18.06.2021 09:30

Mathematics, 18.06.2021 09:30


English, 18.06.2021 09:30

Physics, 18.06.2021 09:30

Advanced Placement (AP), 18.06.2021 09:30


Mathematics, 18.06.2021 09:30


Mathematics, 18.06.2021 09:30

Physics, 18.06.2021 09:30



