What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for...

Chemistry, 25.08.2021 20:10 kyahshayovvu24
What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for water is 1.86 °C/m
Enter rounded answer to three decimal points.
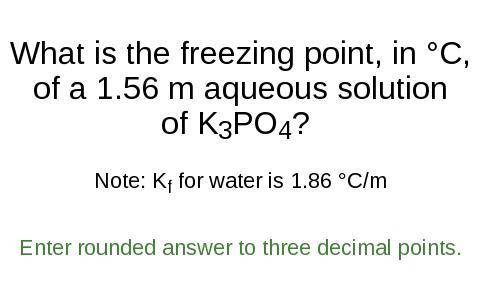

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
Questions

English, 16.02.2021 04:40

Chemistry, 16.02.2021 04:40

Mathematics, 16.02.2021 04:40


Spanish, 16.02.2021 04:40

Mathematics, 16.02.2021 04:40

Mathematics, 16.02.2021 04:50







Mathematics, 16.02.2021 04:50

Mathematics, 16.02.2021 04:50







