
Chemistry, 26.08.2021 23:00 FriendlyDude640
Calculate the percentage of limestone that dissolved from each solution. Start by subtracting the final mass from the initial mass. Divide that number by the initial mass. Then multiply the result by 100 to make it a percent. Use this formula:
% dissolved= initial mass- final mass/ initial mass x 100
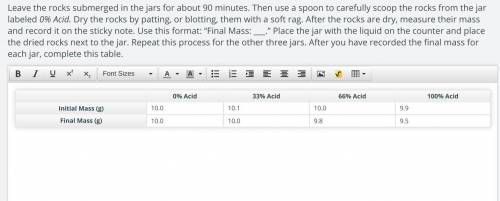
ORIGINAL: 0% Acid 33% Acid 66% Acid 100% Acid
Initial Mass (g) 10.0 10.1 10.0 9.9
Final Mass (g) 10.0 10.0 9.8 9.5
Record the percentage of limestone dissolved in each acid concentration.
Look at attched...

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1


Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Calculate the percentage of limestone that dissolved from each solution. Start by subtracting the fi...
Questions





Computers and Technology, 02.01.2020 21:31


Social Studies, 02.01.2020 21:31







Social Studies, 02.01.2020 21:31



Computers and Technology, 02.01.2020 21:31



Biology, 02.01.2020 21:31



