
Chemistry, 27.08.2021 06:50 amandasantiago2001
Please help I have 15 min to respond this
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
2. Determine the percent yield of MgO for your experiment for each trial.
3. Determine the average percent yield of MgO for the two trials.
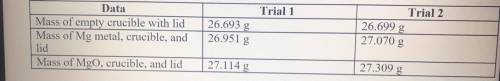

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 11:40
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2
You know the right answer?
Please help I have 15 min to respond this
1. Magnesium is the limiting reactant in this experiment...
Questions

History, 09.11.2021 20:20

Mathematics, 09.11.2021 20:20






English, 09.11.2021 20:20





Social Studies, 09.11.2021 20:20




Chemistry, 09.11.2021 20:20


English, 09.11.2021 20:20

Mathematics, 09.11.2021 20:20



