
Chemistry, 27.08.2021 08:10 ayoismeisalex
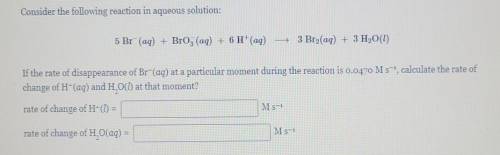
Consider the following reaction in aqueous solution: 5 Br (aq) + Brog (aq) + 6 H+(aq) - 3 Bro(aq) + 3 H2O(l) If the rate of disappearance of Br-(aq) at a particular moment during the reaction is 0.0470 M s-, calculate the rate of change of H-(aq) and H. O() at that moment? rate of change of H- = M s-1 rate of change of H. O(aq) = M s-1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

You know the right answer?
Consider the following reaction in aqueous solution: 5 Br (aq) + Brog (aq) + 6 H+(aq) - 3 Bro(aq) +...
Questions

Engineering, 31.10.2019 00:31



Spanish, 31.10.2019 00:31

Mathematics, 31.10.2019 00:31

Mathematics, 31.10.2019 00:31

Social Studies, 31.10.2019 00:31

History, 31.10.2019 00:31

Mathematics, 31.10.2019 00:31

History, 31.10.2019 00:31












