
Chemistry, 30.08.2021 04:20 barstr9146
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histidine at a) pH 5.5 and b) pH 7.0. A solution is provided, but can you do a step-by-step as I do not quite understand the math that went into this.
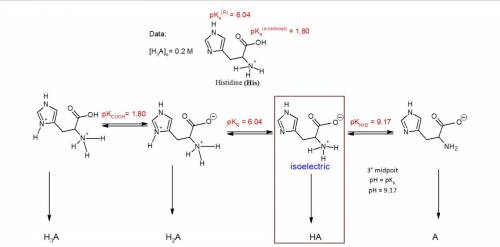
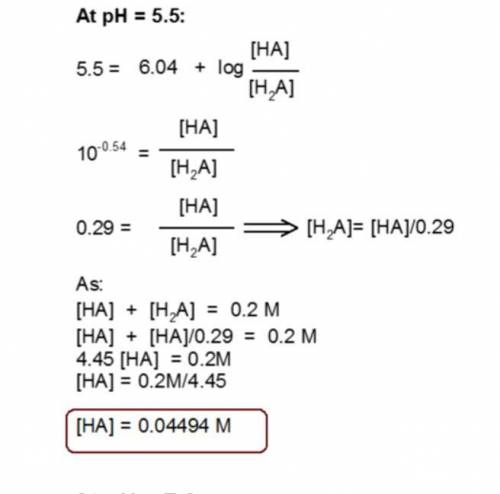

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histid...
Questions


Computers and Technology, 30.11.2021 22:00

Business, 30.11.2021 22:00



Medicine, 30.11.2021 22:00


Mathematics, 30.11.2021 22:00

Biology, 30.11.2021 22:00

Social Studies, 30.11.2021 22:00



Arts, 30.11.2021 22:00

Physics, 30.11.2021 22:00


History, 30.11.2021 22:00


Computers and Technology, 30.11.2021 22:00

Social Studies, 30.11.2021 22:00



