
Chemistry, 31.08.2021 03:30 depinedainstcom
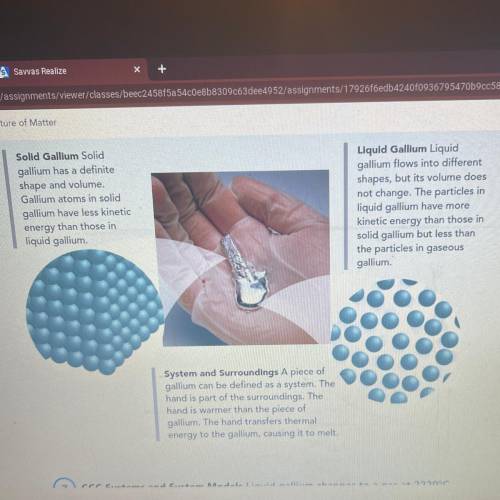
CCC Systems and System Models Liquid gallium changes to a gas at 2229°C.
Describe how a model of gaseous gallium would compare to the model of liquid
gallium shown in the picture.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
CCC Systems and System Models Liquid gallium changes to a gas at 2229°C.
Describe how a model of g...
Questions

Geography, 07.01.2021 01:00

Computers and Technology, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Advanced Placement (AP), 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00



Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


English, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00



