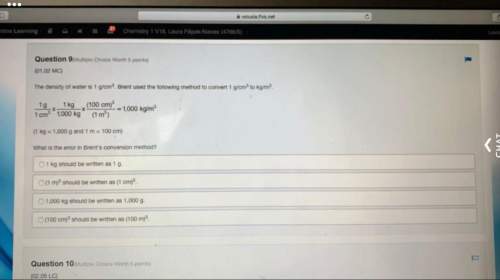
Chemistry, 01.09.2021 19:00 WilliamYES9164
Consider the following reversible reaction.
Upper H subscript 2 upper O (g) double-headed arrow 2 upper H subscript 2 (g) plus upper O subscript 2 (g).
What is the equilibrium constant expression for the given system?
k subscript e q equals StartFraction StartBracket upper H subscript 2 upper O EndBracket over StartBracket upper H subscript 2 EndBracket StartBracket upper O subscript 2 EndBracket EndFraction.
k subscript e q equals StartFraction StartBracket upper H subscript 2 upper O EndBracket superscript 2 over StartBracket upper H subscript 2 EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket EndFraction.
K subscript e q equals StartFraction StartBracket upper H subscript 2 EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket over StartBracket upper H subscript 2 upper O EndBracket EndFraction.
K subscript e q equals StartFraction StartBracket upper H subscript 2 EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket over StartBracket upper H subscript 2 upper O EndBracket superscript 2 EndFraction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3


Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2
You know the right answer?
Consider the following reversible reaction.
Upper H subscript 2 upper O (g) double-headed arrow 2...
Questions

Social Studies, 27.06.2019 10:30


Mathematics, 27.06.2019 10:30



Mathematics, 27.06.2019 10:30



Computers and Technology, 27.06.2019 10:30


Advanced Placement (AP), 27.06.2019 10:30







English, 27.06.2019 10:30