
Chemistry, 02.09.2021 14:00 robert7248
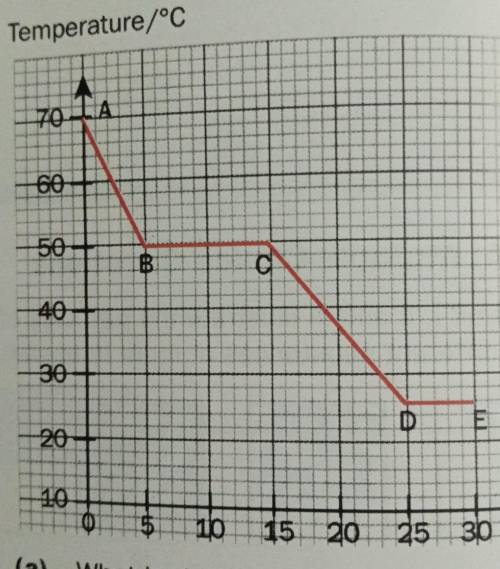
A liquid, X, was allowed to cool in air. The temperature was measured every five secds. The graph below represents the cooling curve of X.
(a) What is the melting point of X?
(b) What is the room temperature? Explain your answer.
(c) X has a boiling point of 128°C. Explain, in terms of the kinetic particle theory, what happens to the particles of X as it is heated from 100°C to 150°C.
(d) In which parts of the graph is energy being given out to the surroundings?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2



Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
A liquid, X, was allowed to cool in air. The temperature was measured every five secds. The graph be...
Questions


Mathematics, 15.07.2019 05:00

Arts, 15.07.2019 05:00


Mathematics, 15.07.2019 05:00

Mathematics, 15.07.2019 05:00


English, 15.07.2019 05:00


Social Studies, 15.07.2019 05:00

Biology, 15.07.2019 05:00



History, 15.07.2019 05:00


English, 15.07.2019 05:00

English, 15.07.2019 05:00

English, 15.07.2019 05:00

Health, 15.07.2019 05:00



