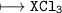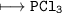---
An atom of element X contains 15 electrons and 16 neutrons.
(a). (I) State the mass...

Chemistry, 05.09.2021 07:50 jamisoncameron000
---
An atom of element X contains 15 electrons and 16 neutrons.
(a). (I) State the mass number of X.
(ii) Write the electronic
configuration of X.
(b) (I) Write the formula of a chloride of X. pliz , it's urgent.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Questions












Chemistry, 31.07.2019 07:10






Mathematics, 31.07.2019 07:10

World Languages, 31.07.2019 07:10

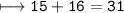
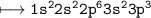 .
.