
Chemistry, 08.09.2021 02:40 accounting73
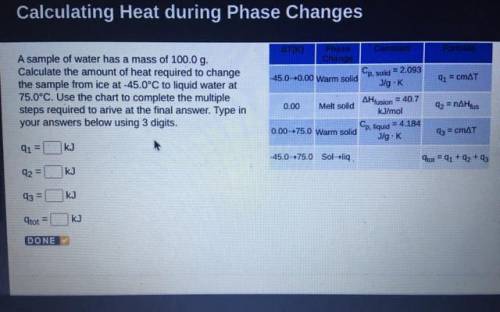
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the sample from ice at -45.0°C to liquid water at
75.0°C. Use the chart to complete the multiple
steps required to arive at the final answer. Type in
your answers below using 3 digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

You know the right answer?
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the...
the...
Questions

Mathematics, 25.01.2021 20:10

Mathematics, 25.01.2021 20:10





Mathematics, 25.01.2021 20:10

Mathematics, 25.01.2021 20:10




Health, 25.01.2021 20:10


Biology, 25.01.2021 20:10


Social Studies, 25.01.2021 20:10


Mathematics, 25.01.2021 20:10

English, 25.01.2021 20:10

Mathematics, 25.01.2021 20:10



