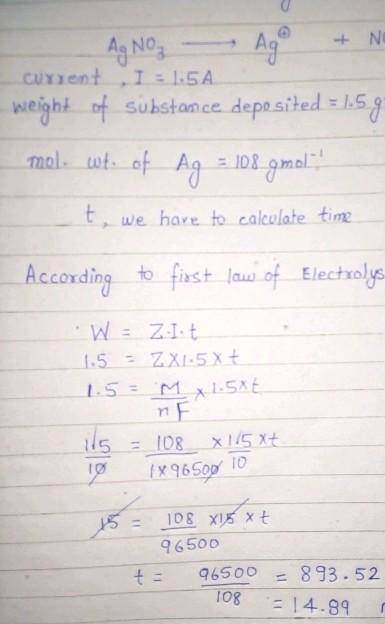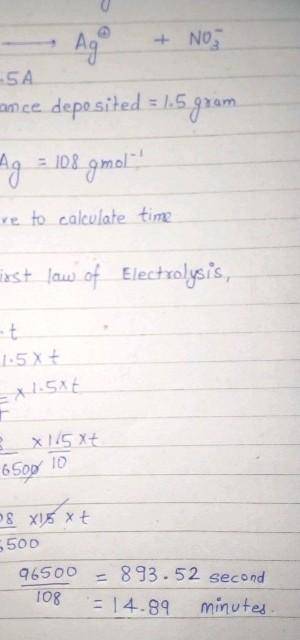Chemistry, 10.09.2021 17:20 rachelshryock551
calculate the amount of current that must flow for 50 minutes to a solution of silver trioxonitrate (V) solution to deposit 2 mole of silver. (Ag=108, Faraday's constant=96500C mol¯¹)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3


Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
calculate the amount of current that must flow for 50 minutes to a solution of silver trioxonitrate...
Questions


Mathematics, 01.02.2021 01:00


Mathematics, 01.02.2021 01:00



Mathematics, 01.02.2021 01:00

Computers and Technology, 01.02.2021 01:00

English, 01.02.2021 01:00





Mathematics, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00

English, 01.02.2021 01:00

English, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00