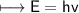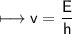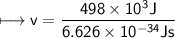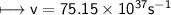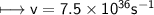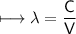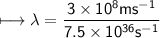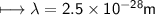Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2


Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
It takes 498. kJ/mol to break an oxygen-oxygen double bond. Calculate the maximum wavelength of ligh...
Questions


Computers and Technology, 30.06.2020 07:01

Mathematics, 30.06.2020 07:01

Mathematics, 30.06.2020 07:01


History, 30.06.2020 07:01













Mathematics, 30.06.2020 07:01

English, 30.06.2020 07:01