
Chemistry, 12.09.2021 19:10 annabanana1298
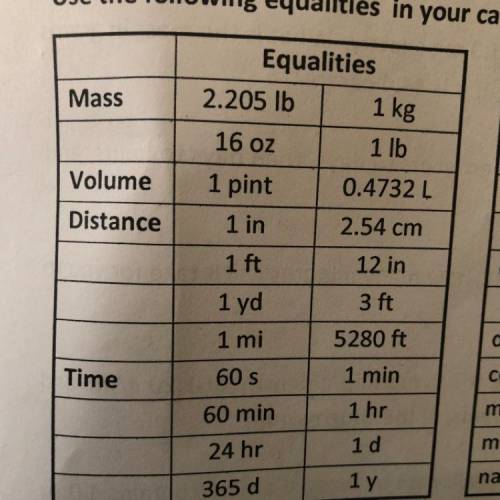
A sunscreen preparation contains 2.50% benzyl salicylate by mass. If a tube contains 4.0 oz
of sunscreen, how many kilograms of benzyl salicylate are needed to manufacture 325 tubes
of sunscreen?
We must solve this through a multi-step dimensional analysis process, but I am having trouble since there is a percentage present in the question. Any help would be appreciated!

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
A sunscreen preparation contains 2.50% benzyl salicylate by mass. If a tube contains 4.0 oz
of sun...
Questions

Mathematics, 08.10.2020 07:01


History, 08.10.2020 07:01




Mathematics, 08.10.2020 07:01

Mathematics, 08.10.2020 07:01

Mathematics, 08.10.2020 07:01



Mathematics, 08.10.2020 07:01


Arts, 08.10.2020 07:01

Computers and Technology, 08.10.2020 07:01







