
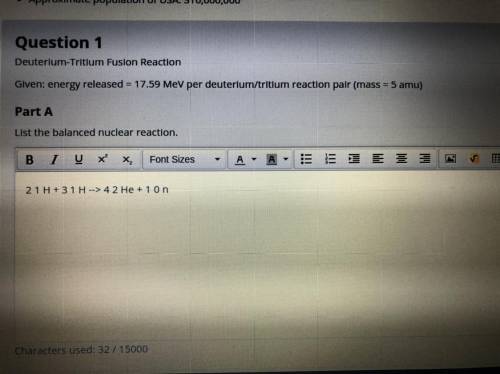
Part A. Deuterium-tritium fusion reaction. Given: energy released = 17.59 MeV per deuterium/tritium reaction pair (mass = 5 amu)
Part B. Determine the energy released per kilogram of fuel used. Given MeV per reaction, calculate energy in joules per kilogram of reactants. Consider 1 mole of tritium plus 1 mole of deuterium yo be a mole of “reactions” (total molar mass = 5 grams)
Part C. Determine the mass of fuel required for the expected energy consumption in the United States for the next 10 years. Energy used per person per year in the United States = 3.5 x 10^11 joules. Base your calculations on a current population of 310,000,000

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3
You know the right answer?
Part A. Deuterium-tritium fusion reaction. Given: energy released = 17.59 MeV per deuterium/tritium...
Questions

Mathematics, 12.08.2020 06:01




Mathematics, 12.08.2020 06:01















Mathematics, 12.08.2020 06:01



