
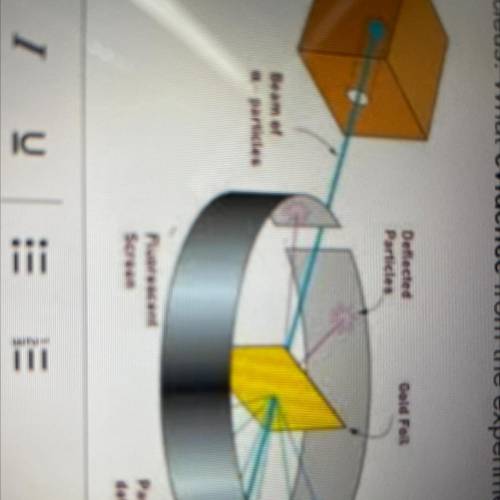
PLS HELP ME After completing the Gold Foil Experiment, Ernest Rutherford proposed that the positive charge of an atom was concentrated in a dense central core or nucleus. What evidence from the experiment did he use to support this idea? Your answer should reference the image.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
PLS HELP ME After completing the Gold Foil Experiment, Ernest Rutherford proposed that the positive...
Questions


Biology, 03.05.2021 18:50


English, 03.05.2021 18:50




Mathematics, 03.05.2021 18:50

Mathematics, 03.05.2021 18:50



Mathematics, 03.05.2021 18:50

Chemistry, 03.05.2021 18:50




Mathematics, 03.05.2021 18:50


Mathematics, 03.05.2021 18:50

Health, 03.05.2021 18:50



