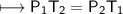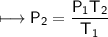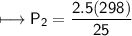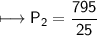Chemistry, 17.09.2021 08:30 aischa282005
A spray paint can with a pressure of 2.50 atm is trapped inside a burning
factory. Its initial temperature is 25°C. What is the final pressure of the can
if the fire reaches a temperature of 635°C? Assume that the volume of the
can remains constant!

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
A spray paint can with a pressure of 2.50 atm is trapped inside a burning
factory. Its initial tem...
Questions

Mathematics, 22.09.2019 21:20






Mathematics, 22.09.2019 21:30


Business, 22.09.2019 21:30


Business, 22.09.2019 21:30


English, 22.09.2019 21:30

Biology, 22.09.2019 21:30



World Languages, 22.09.2019 21:30