
Chemistry, 18.09.2021 01:00 annemcnair217
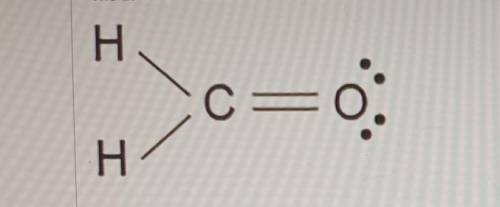
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 points)
O Oxygen is the least electronegative of the three atoms.
O Carbon has a total of four bonded pairs of electrons around it.
O Oxygen has four pairs of non-bonding innermost shell electrons.
O Carbon has an incomplete octet as it transfers an electron to each hydrogen.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 p...
Questions


Mathematics, 30.10.2019 00:31


History, 30.10.2019 00:31


English, 30.10.2019 00:31




Advanced Placement (AP), 30.10.2019 00:31

Chemistry, 30.10.2019 00:31

History, 30.10.2019 00:31



Business, 30.10.2019 00:31

History, 30.10.2019 00:31



World Languages, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31



