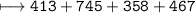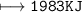Chemistry, 19.09.2021 14:00 CuteDoggo1828
Use the reaction and bond information to answer the question.
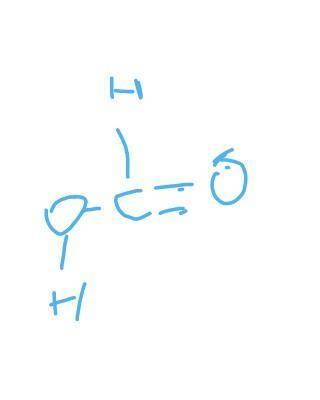
H2 + CO2 → CH2O2
Reactant bond energies: H–H is 432 kJ/mol, C=O is 799 kJ/mol
Product bond energies:
C–H is 413 kJ/mol, C=O is 745 kJ/mol
C–O is 358 kJ/mol, O–H is 467 kJ/mol
How much energy will be given off by the products?
1,570 kJ
1,983 kJ
1,238 kJ
1,516 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Use the reaction and bond information to answer the question.
H2 + CO2 → CH2O2
Reactan...
Reactan...
Questions

Geography, 27.10.2020 14:00

Social Studies, 27.10.2020 14:00

Social Studies, 27.10.2020 14:00

English, 27.10.2020 14:00


Mathematics, 27.10.2020 14:00

Computers and Technology, 27.10.2020 14:00


Physics, 27.10.2020 14:00

English, 27.10.2020 14:00

Health, 27.10.2020 14:00

Physics, 27.10.2020 14:00

Chemistry, 27.10.2020 14:00


Mathematics, 27.10.2020 14:00

Mathematics, 27.10.2020 14:00

Biology, 27.10.2020 14:00


Chemistry, 27.10.2020 14:00

English, 27.10.2020 14:00