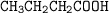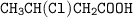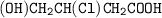Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
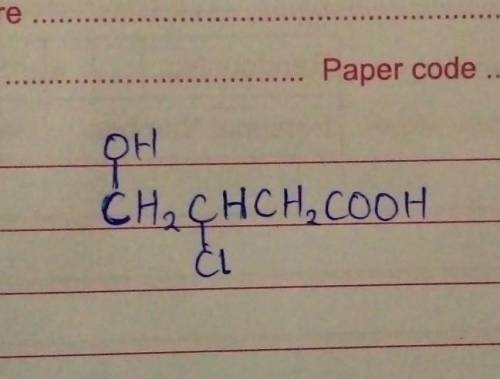
Draw the structure of ;
3-chloro-4-hydroxylbutanoic acid....
3-chloro-4-hydroxylbutanoic acid....
Questions

Mathematics, 28.08.2019 21:50



Mathematics, 28.08.2019 21:50



Biology, 28.08.2019 21:50

History, 28.08.2019 21:50



Biology, 28.08.2019 21:50




Health, 28.08.2019 21:50

History, 28.08.2019 21:50

English, 28.08.2019 21:50



Computers and Technology, 28.08.2019 21:50