
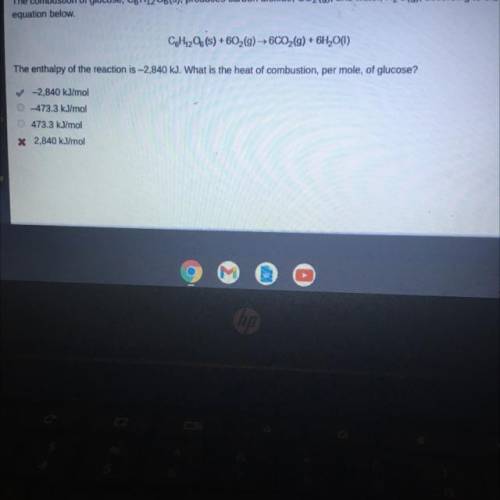
The combustion of glucose, C6H1206(s), produces carbon dioxide, CO2(g), and water, H2O(g), according to the
equation below.
CoH1206(s) +602(g) → 6CO2(g) + 6H2O(1)
The enthalpy of the reaction is -2,840 kJ. What is the heat of combustion, per mole, of glucose?
✓-2,840 kJ/mol
473.3 kJ/mol
473.3 kJ/mol
* 2,840 kJ/mol
See

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

You know the right answer?
The combustion of glucose, C6H1206(s), produces carbon dioxide, CO2(g), and water, H2O(g), according...
Questions





Mathematics, 20.08.2019 02:30

History, 20.08.2019 02:30

Biology, 20.08.2019 02:30

Chemistry, 20.08.2019 02:30

Mathematics, 20.08.2019 02:30


English, 20.08.2019 02:30

Mathematics, 20.08.2019 02:30



Mathematics, 20.08.2019 02:30

Mathematics, 20.08.2019 02:30



Mathematics, 20.08.2019 02:30



