
Chemistry, 22.09.2021 02:30 gwendallinesikes
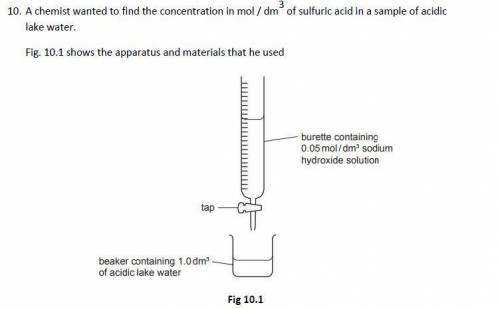
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water contained in a beaker until the acid had just been neutralised
The chemist found that it required 12.5 cm3 of 0.05 mol / dm3 sodium hydroxide solution to neutralise the acid
a. State the number of moles of sodium hydroxide which are dissolved in 1.0 dm3 of the sodium hydroxide solution
b. Calculate the number of moles of sodium hydroxide which are dissolved in 12.5 cm3 of the sodium hydroxide solution. Show your workings.
Show your working.
c. The balanced equation for the neutralisation reaction is
2NaOH + H2SO4 → Na2SO4 + 2H2O
Calculate the number of moles of sulfuric acid which were contained in 1.0 dm3 of acidic lake water.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water co...
Questions







Mathematics, 12.12.2019 04:31









Mathematics, 12.12.2019 04:31



Social Studies, 12.12.2019 04:31

History, 12.12.2019 04:31



