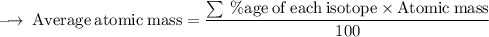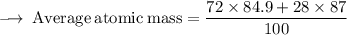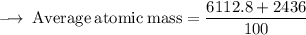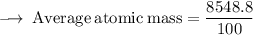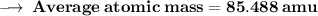Chemistry, 22.09.2021 14:00 demetricejames
Element X has two isotopes. If 72.0% of the element has an isotope mass of 84.9 atomic mass units, and 28.0% of the element has an isotopic mass of 87.0 atomic mass units, the average atomic mass of element X is numerically equal to

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
Element X has two isotopes. If 72.0% of the element has an isotope mass of 84.9 atomic mass units, a...
Questions


Mathematics, 20.08.2019 00:40




History, 20.08.2019 00:40



Social Studies, 20.08.2019 00:40

Biology, 20.08.2019 00:40


Mathematics, 20.08.2019 00:40



Mathematics, 20.08.2019 00:40


Mathematics, 20.08.2019 00:40

English, 20.08.2019 00:40

Chemistry, 20.08.2019 00:40