
Chemistry, 24.09.2021 07:50 alicciardone01
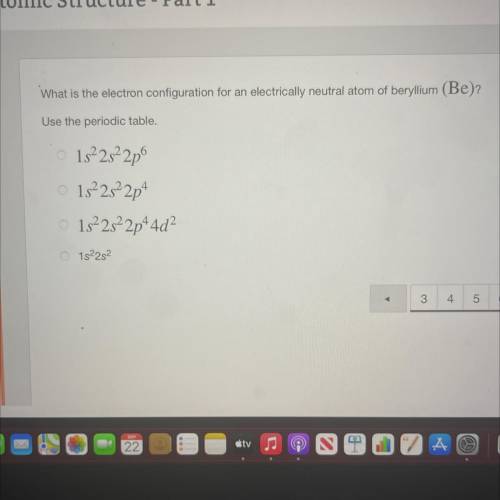
What is the electron configuration for an electrically neutral atom of beryllium (Be)?
Use the periodic table.
1s²2s22p
1522s22p+
1s22s22p+4d2
O 15²25²

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
What is the electron configuration for an electrically neutral atom of beryllium (Be)?
Use the per...
Questions


Mathematics, 03.09.2020 18:01


Health, 03.09.2020 18:01

Spanish, 03.09.2020 18:01










English, 03.09.2020 18:01

English, 03.09.2020 18:01

Mathematics, 03.09.2020 18:01





