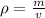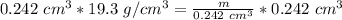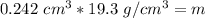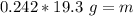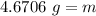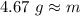. The density of gold is


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Calculate the mass of a sample of gold that
occupies 0.242 cm3
. The density of gold is
. The density of gold is
Questions


Mathematics, 01.12.2020 03:20


Physics, 01.12.2020 03:20

Mathematics, 01.12.2020 03:20

Mathematics, 01.12.2020 03:20


Mathematics, 01.12.2020 03:20

Mathematics, 01.12.2020 03:20

Mathematics, 01.12.2020 03:20



English, 01.12.2020 03:20


Health, 01.12.2020 03:20



Mathematics, 01.12.2020 03:20