CH3CO2H is a base, and H3O+ is its conjugate acid.

Chemistry, 25.09.2021 17:10 22savage2017
In the buffer solution
A)
CH3CO2H is a base, and H3O+ is its conjugate acid.
B)
H3O+ is an acid, and CH3CO2 – is its conjugate base.
C)
H3O+ is an acid, and CH3CO2H is its conjugate base.
D)
CH3CO2H is an acid, and CH3CO2 – is its conjugate base.
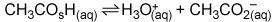

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
In the buffer solution
A)
CH3CO2H is a base, and H3O+ is its conjugate acid.
CH3CO2H is a base, and H3O+ is its conjugate acid.
Questions




Mathematics, 19.09.2020 01:01

History, 19.09.2020 01:01


Social Studies, 19.09.2020 01:01

Business, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01

Physics, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

Biology, 19.09.2020 01:01









