
Chemistry, 27.09.2021 01:00 mrsdeanwinchester18
Cho dung dịch Ba(OH)2 dư vào 50ml dung dịch X chứa các ion NH4+; SO42-; NO3- đun nóng thì có 11,65g kết tủa xuất hiện và có 4,48lít khí Y thoát ra. Nồng độ mỗi muối trong dung dịch X là
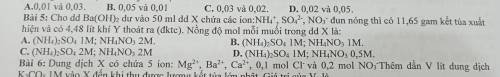

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1
You know the right answer?
Cho dung dịch Ba(OH)2 dư vào 50ml dung dịch X chứa các ion NH4+; SO42-; NO3- đun nóng thì có 11,65g...
Questions


Spanish, 19.03.2020 00:43


Mathematics, 19.03.2020 00:43

Biology, 19.03.2020 00:43




Law, 19.03.2020 00:43






English, 19.03.2020 00:43



Mathematics, 19.03.2020 00:43




