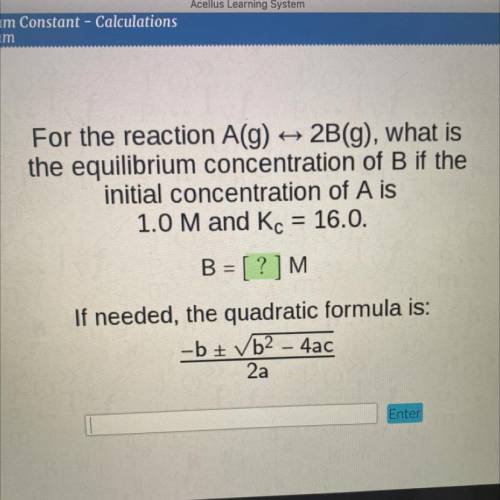
For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial...

For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial concentration of A is
1.0 M and Kc = 16.0.
B = [?]M
If needed, the quadratic formula is:
-b + b2 - 4ac
2a
PLEASE HELP IM SO CONFUSED AND NO LESSON VIDEOS ARE HELPFULL
ILL RATE YOU BRAINLIEST

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Questions

Mathematics, 02.02.2020 22:55



Social Studies, 02.02.2020 22:55

Biology, 02.02.2020 22:55



History, 02.02.2020 22:55

Geography, 02.02.2020 22:55


Social Studies, 02.02.2020 22:55


Business, 02.02.2020 22:55

Mathematics, 02.02.2020 22:55



Mathematics, 02.02.2020 22:55

Physics, 02.02.2020 22:55




