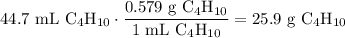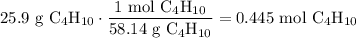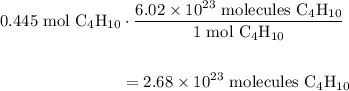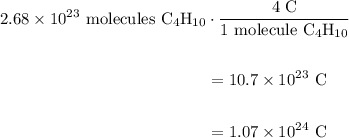Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
A beaker contains 44.7 mL of butane ( C4H10, density is 0.579 g/mL).
Determine how many C atoms th...
Questions

Mathematics, 22.06.2021 05:30

Mathematics, 22.06.2021 05:30


Mathematics, 22.06.2021 05:30



Chemistry, 22.06.2021 05:30




Mathematics, 22.06.2021 05:30

Mathematics, 22.06.2021 05:30

Mathematics, 22.06.2021 05:30



Mathematics, 22.06.2021 05:30

English, 22.06.2021 05:30

Biology, 22.06.2021 05:30

Mathematics, 22.06.2021 05:30