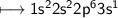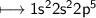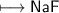6. Neon has 10 electrons - 2 in the inner shell and 8 in the outer shell. This arrangement of 8 electrons in the
outer shell is extremely stable and makes neon inert, with a valence of zero. Sodium has 11 electrons - 2
in the first shell, 8 in the next shell, and I in the outer shell. Fluorine has 9 electrons - 2 in the first shell
and 7 in the outer shell. How could sodium and fluorine from a compound in which both elements would
be like neon with 8 electrons in the outer shell?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1


Chemistry, 23.06.2019 13:00
What mass of ca(oh)2 is needed to make 1250ml of a .75m solution?
Answers: 3
You know the right answer?
6. Neon has 10 electrons - 2 in the inner shell and 8 in the outer shell. This arrangement of 8 elec...
Questions

Arts, 22.04.2021 18:00


Mathematics, 22.04.2021 18:00


Mathematics, 22.04.2021 18:00


Biology, 22.04.2021 18:00

Business, 22.04.2021 18:00

Mathematics, 22.04.2021 18:00

History, 22.04.2021 18:00


Mathematics, 22.04.2021 18:00

Mathematics, 22.04.2021 18:00

Mathematics, 22.04.2021 18:00


Mathematics, 22.04.2021 18:00


English, 22.04.2021 18:00

Spanish, 22.04.2021 18:00