
Chemistry, 29.09.2021 20:30 Katiecool290
STEM 11
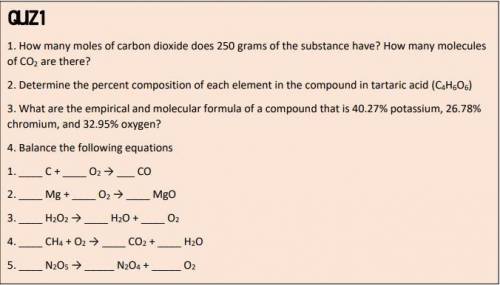
1.How many moles of carbon dioxide does 250 grams of the substance have? How many molecules of CO2 are there?
2. Determine the percent composition of each element in the compound in tartaric acid (C4H6O6)
3. What are the empirical and molecular formula of a compound that is 40.27% potassium, 26.78%
chromium, and 32.95% oxygen?
4. Balance the following equations
1. _ C + _ O2 → ___ CO
2. _ Mg + _ O2 → _ MgO
3. _ H2O2 → _ H2O + _ O2
4. _ CH4 + O2 → _ CO2 + _ H2O
5. _ N2O5 → _ N2O4 + _ O2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
STEM 11
1.How many moles of carbon dioxide does 250 grams of the substance have? How many molecule...
Questions

Mathematics, 23.01.2020 05:31

Biology, 23.01.2020 05:31

Social Studies, 23.01.2020 05:31

Mathematics, 23.01.2020 05:31

Health, 23.01.2020 05:31


Mathematics, 23.01.2020 05:31


Mathematics, 23.01.2020 05:31





English, 23.01.2020 05:31

Mathematics, 23.01.2020 05:31


Mathematics, 23.01.2020 05:31



Mathematics, 23.01.2020 05:31



