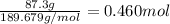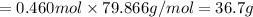Chemistry, 29.09.2021 21:20 Rflaig1129841
When TiCl4 (s) reacts with H20 (), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g of
titanium (IV) chloride present, with water in excess, how much solid titanium (IV) oxide (in grams)
could theoretically be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1


Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

You know the right answer?
When TiCl4 (s) reacts with H20 (), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g...
Questions


Chemistry, 22.11.2020 05:40

English, 22.11.2020 05:40

English, 22.11.2020 05:40



Mathematics, 22.11.2020 05:40




Social Studies, 22.11.2020 05:40

Mathematics, 22.11.2020 05:40

Mathematics, 22.11.2020 05:40

Computers and Technology, 22.11.2020 05:40

English, 22.11.2020 05:40

Mathematics, 22.11.2020 05:40

Health, 22.11.2020 05:40



Biology, 22.11.2020 05:40